Tandem Diabetes Care Plans for Future with Technology Choices
In the coming years, Tandem Diabetes Care has ambitious plans to introduce the next version of its tubed t:slim insulin pump, a series of three smaller devices to reduce and eventually eliminate tubes completely, and features allowing users to fully control their insulin pump and even deliver bolus insulin with their smartphones.
The San Diego, California-based company revealed all of this at its first-ever R&D Day on Dec. 6, 2021, mapping out its 5-year pipeline plan for new technology.
While medtech timelines often slip, given corporate priorities and the Food and Drug Administration (FDA) review process, Tandem expects that it will be able to develop and launch most — if not all — of these new products on a rolling scale between 2022 and 2027.
"As a diabetes care company, we realize there is not a one-size-fits-all solution to managing this complex condition," Tandem CEO John Sheridan said. "'Positively different' is a sum total of our brand... As we look to the future of our hardware strategy, we are moving away from offering a single platform and will be emphasizing choice."
It's likely that as these new devices and mobile data options are launched, they will be compatible with Tandem's existing products including its Basal-IQ and Control-IQ algorithms, and will continue to integrate with the latest Dexcom continuous glucose monitor system.
Here's a look at the new products in development:
Smartphone insulin dosing and device control
Pieces of Tandem's tech plan are already in the works, with a key first step already submitted to the FDA.
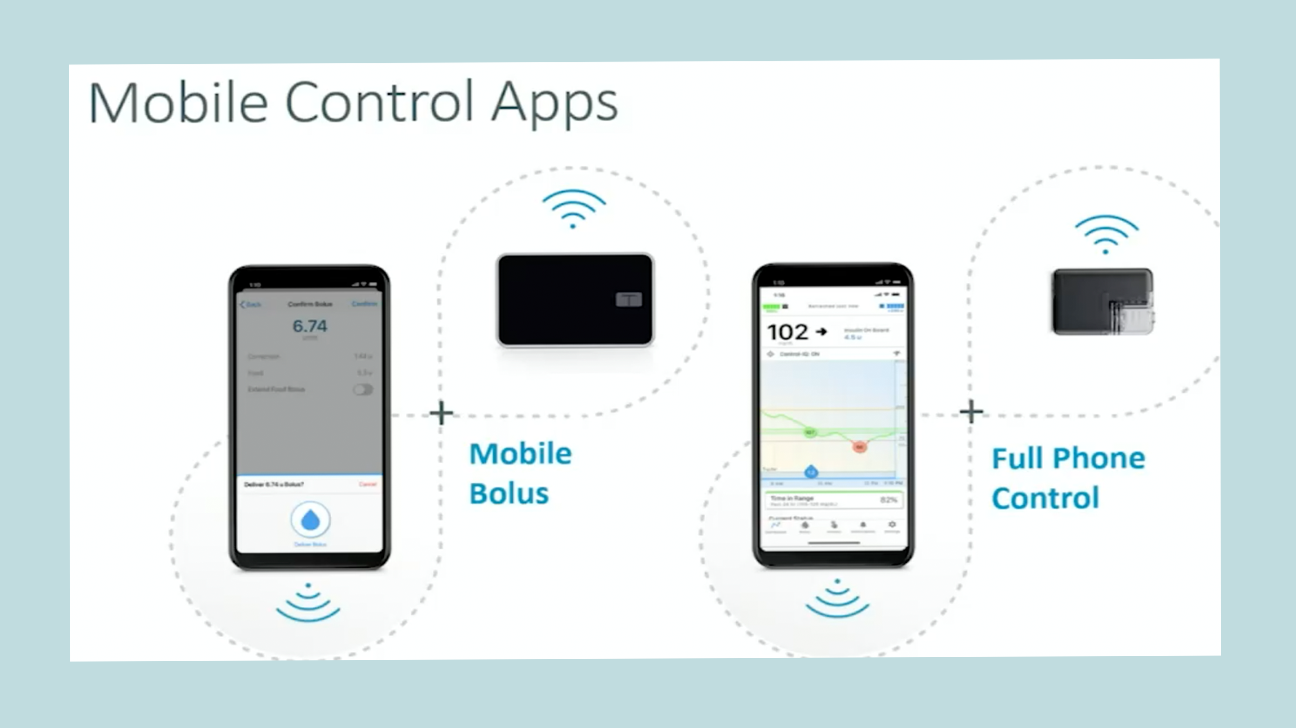
There are two parts to their mobile data pipeline:
Bolus-by-phone. Tandem asked regulators in late 2020 to OK its mobile bolusing by phone app function, which would allow Tandem customers to use their iPhone or Android smartphone apps to dose insulin without needing to take out the pump itself. The company had hoped for approval and launch in 2021, but delays related to the COVID-19 pandemic have caused an FDA backlog, so review is taking longer than expected. [UPDATE: Tandem announced on Feb. 16, 2022 that it had received FDA clearance for its mobile bolusing feature for both iOS and Android, and it's expected to launch in summer 2022.]
Full device control by phone. This would come down the road, going beyond just bolusing insulin remotely. It would allow for other features that include changing basal (background insulin) rates, turning alerts and alarms on or off, suspending and resuming insulin delivery, and more directly from your phone. The company has not yet named a specific timeline for this to be submitted to regulators.
Tandem believes FDA clearance for the mobile bolusing function could come "any day now," and is planning for an early 2022 launch. There is no official word yet on whether existing Tandem pumpers will need to update or download an entirely new mobile app for their iOS or Android devices, to accommodate this new capability. That key detail will be shared once FDA's requirements are known.
Tandem Mobi (formerly t:sport mini-pump)

The company has been developing a hybrid micro-pump of sorts, roughly half the size of the current t:slim X2 pump and without any display screen at all. Initially referred to as the t:sport and now branded as Tandem Mobi, this mini-pump has both a short, 4-inch tubing with the trademark "pigtail" connector that goes to an infusion set as well as adhesive on the back to stick onto the body — so it can be worn either way.
Here is a rundown of the Tandem Mobi outlined at the recent R&D Day:
- roughly 50 percent of the t:slim X2 size
- will hold 200 units of insulin in the cartridge
- operated solely by an iOS or Android smartphone
- allows for wireless charging
- has a bolus button on the side of the device
- waterproof
- compatible with the latest "iCGM" devices like the Dexcom G6
- has an embedded Automated Insulin Delivery (AID) algorithm to be compatible with Tandem Control-IQ features
- compatible with current Tandem infusion sets as well as a future 4-inch set in development
DiabetesMine got a first glimpses of the t:sport prototype at the company’s San Diego headquarters in 2017. Tandem had planned to submit this device to the FDA in 2020, but the pandemic delayed the clinical trial, and it's now waiting on the new mobile app with remote bolusing feature.

Tandem now plans to file Mobi with the FDA after it secures regulatory approval for the mobile bolusing app feature, and finalizes any necessary revisions or clinical work needed from there. That could be as early as the second half of 2022, but there's no solid timeline yet.
Enhancements to Tandem's Control IQ
Aside from brand new product offerings, Tandem is also planning to improve its existing software with new features. Specifically, that may involve lower glucose targets (ie 100 mg/dL instead of the current 110/112 mg/dL), which is something many Tandem tech users want to see.
"We are driving innovation in our algorithms, emphasizing automation, personalization, and simplification, all intended to continue to improve therapeutic outcomes and provide a positive patient experience characterized by simplicity and ease of use," the Tandem product pipeline says. "Examples of our efforts to provide enhanced personalization include alternative targets, settings optimizations, and enhanced exercise options. Examples of our efforts to provide greater ease of use include adaptations, smarter alerts, and new signal integrations."
Tandem also plans to pursue expanded Control IQ indications for kids as young as 2 years old, as well as those with type 2 diabetes.
There is no public timeline on these enhancements, but it seems likely that they'll be happening concurrently with other clinical trial research and regulatory discussion on everything else in Tandem's near-term pipeline. Hopefully, we can see some of these improvements by early 2023.
Tandem t:slim X3
This is Tandem's third generation of its t:slim pump, which soon marks its 10-year launch anniversary from August 2012. The second version came in 2016 with the t:slim X2, the first to be compatible with a CGM.
There is not currently much detail on what will change with the X3, but we were informed that it will have the same basic form factor with a color touchscreen as the current generation. Features will include:
- holds 300 units of insulin
- enhanced technology
- refreshed user interface
- increased battery life
- wireless software updates
This new model will follow approval of the first-generation of Tandem Mobi, expected at some point in 2022 or early 2023.
Tandem Mobi Tubeless pump

This new device is the second of a three-part plan moving toward a full patch pump without any tubes. While the first version of Tandem Mobi will be a hybrid with short tubing, the second iteration will go tubeless.
Instead of pump tubing and the t:connect "pigtail" connector attaching to an infusion set, this Mobi Tubeless will replace that infusion set with a "disposable on-body kit." Conceptual designs appear to show the Tandem Mobi Tubeless adhering directly onto the body, with a black rectangle shape and removable insulin cartridge. As it uses the same Mobi pump design, it will likely also hold 200 units.
Fully disposable pump patch
Finally, Tandem will develop what it describes as a fully-disposable pump patch. There wasn't much detail provided on this future product, as Tandem says it's very early in the development process. A conceptual image shows a thin white patch-like device adhered to the upper arm, but that may only be a prototype placeholder until the concept is more fully developed in the coming years.
No doubt, with these tubeless patch pump devices, Tandem has in mind going up against its competitor — the tubeless Omnipod made by Insulet. That's not surprising, given the excitement over the sophisticated new Omnipod 5 system expected to become available in 2022 as the first tubeless closed loop system.
Infusion sets and more
Tandem is also expecting to launch new pump infusion sets as it moves forward with these next-generation devices. In addition, the company says it's working to allow its devices to use higher concentration insulin, as well as more personalized settings and management options for those with diabetes.
Options are certainly the key, coupled with affordability and access. Way to go on bringing more flexibility and choice for people whose lives depend on insulin, Tandem!
----------------------------------------------
Originally reported by Mike Hoskins at DiabetesMine

Comments
Never had a problem with Tandem T2 Slim/Dexcom G6 combo until I installed the Dexcom G6 app on my iPhone.
Same night, while receiving ongoing "loss of signal" alerts, my pump delivered a huge amount of insulin at the same time I was crashing and receiving Urgent Low alarms. Then, same thing happened again, 2 weeks later.
I have low blood sugars all the time, but they're always self induced and easy to trouble shoot.
No clue WHAT caused this. My pump was running on Moonlight 7.4, which IS on Tandem's URGENT UPDATE ALERT list, yet Tandem insists not having updated to the latest OS has nothing to do with the issue.
The diagnosis: Failed Dexcom Transmitter AND Failed Dexcom Sensor. I've had plenty of sensors and a few transmitters fail, before, and while that might have come into play, I don't buy it. The behavior of this pump was so far out in left field from ANYTHING I've experienced, it's not logical. (BTW, it's my opinion the Dexcom G6 is INCREDIBLE.)
The only thing NEW was putting the G6 Mobile App on my phone. NEVER had a pump/cgm combo malfunction (and I lived through many recalls with MiniMed) even remotely similar to this situation.
Both nights were the closest I've ever come to biting it, since I started wearing pumps, many MANY years ago. Yet, the party line at both companies is, "Control IQ worked as designed."