Highlights of the American Diabetes Association’s 2021 Annual Meeting
The American Diabetes Association's annual conference, known as the ADA Scientific Sessions, is always the biggest diabetes event of the year, and 2021 marked the second time this 5-day congress was held completely online because of the lingering COVID-19 pandemic.
When held in person, the conference normally convenes roughly 16,000 physicians, researchers, and diabetes industry experts from across the globe. This 81st annual event drew 11,600 people from 119 countries between June 25 and 29 — slightly lower than the 12,527 registered attendees for the 2020 virtual event. For both, the event organizers expected more people to tune in afterward, thanks to the recorded online sessions being made available for up to 3 months following the conference.
Despite its virtual nature, this year's conference included nearly 200 presentations with more than 900 presenters on any range of topics. And to top it off, there were roughly 1,100 research posters delving into the latest science in diabetes. You can catch up on some of the action by searching hashtag #ADA2021.
Many of the big themes this year were extensions of what we saw in 2020 with the first-ever virtual SciSessions, but with even deeper focus. Below is our team's summary of conference highlights.
COVID-19 and diabetes
Of course, the novel coronavirus that took the world to its knees was a main focal point and recurring theme in a large majority of research presented at this year's Scientific Sessions.
Whether the topic officially had to do with COVID-19 or not, this was on everyone's mind — from telehealth to research delays because of shutdowns, hospitalizations, etc. New research highlighted how people with underlying health conditions are 6 times more likely to die of COVID-19, and diabetes is the second most reported condition tied to those deaths in the U.S.
"Seeing the devastating impact of the pandemic on people with diabetes, the ADA is emboldened to work even harder to lead the fight against diabetes," said the ADA's chief scientific and medical officer, Dr. Robert Gabbay. "Our mission is reinforced by researchers from around the globe committed to closely studying specific impacts and interventions to help people living with diabetes during this COVID-19 era."
Research from the T1D Exchange presented at ADA showed that among people with type 1 diabetes (T1D), use of diabetes technology lowered the risk of adverse outcomes with COVID-19. That point was emphasized throughout the conference, though it was offset by the common barriers of access and affordability issues — as well as racial and ethnic disparities in diabetes technology use.
One disturbing research presentation illustrated how type 2 diabetes (T2D) in children had skyrocketed during the COVID-19 pandemic. More pediatric patients were hospitalized between March and December 2020 than in the previous year. It also shows that stay-at-home orders resulting from COVID-19 exacerbated T2D risk for children overall, largely because of limited physical activity, more screen time and sedentary behaviors, disrupted sleep, and higher intake of processed foods and differing eating patterns during the day. A notable stat presented in one session showed that 1 in 4 PWDs in America reported the pandemic had interfered with their ability to obtain healthy food.
"While our study examined hospital admissions for type 2 diabetes in children at one center, the results may be a microcosm of what is happening at other children’s hospitals across the country," said Dr. Daniel S. Hsia of the Pennington Biomedical Research Center in Baton Rouge, LA. "Unfortunately, COVID-19 disrupted our lives in more ways than we realize. Our study reinforces the importance of maintaining a healthy lifestyle for children even under such difficult circumstances."
Another study conducted in October 2020 showed that 1 in 5 adults with diabetes reported anxiety or depression. Nearly half of adults (or 47 percent) with T1D reported moderate to severe distress compared with only 11 percent of adults with T2D. That research came from Dr. Sarah C. Westen at the University of Florida, and she told attendees that it meant PWDs with these pandemic-related psychosocial concerns needed follow-up diabetes care aimed at mental health.
Overall, the most common themes were that COVID-19 led to increased health anxiety, limited social interaction, and routine disruption. Many presenters also emphasized the need for more longitudinal research to better understand how these psychosocial factors specifically impacted diabetes management during the pandemic.
"While we are beyond eager to return to 'normal' and are well aware of the devastation that continues to occur because of COVID, we hope to take these silver linings, learn from them, and continue to implement things that we found particularly helpful that resulted out of necessity because of the pandemic," said Catlin Dennis, MPH, of the Oregon-based Novel Interventions in Children’s Healthcare (NICH) at Doernbecher Children's Hospital. She presented in a session titled "When COVID-19 Clashes with Diabetes."
Health inequities and racism
Not surprisingly, racial disparities and inequities within diabetes care were a focal point at the ADA conference as well. Many presenters noted that existing disparities were brought to light quite glaringly during the height of COVID-19.
In August 2020, the ADA published a "Health Equity Bill of Rights" that included statements on access to insulin and other diabetes meds, affordable healthcare, and ensuring that PWDs are able to be free from stigma and discrimination. As of April 2021, the ADA is encouraging scientists to apply for grants to conduct research touching on the impact of disparities in diabetes care.
“We can’t improve the health of all Americans without first addressing health inequities in our healthcare system. It’s crucial that we take a groundwater approach to solving these problems so that the solutions are both sustainable and effective. We have an obligation to dismantle these inequities and eliminate the devastating impact they have on families and communities,” ADA CEO Tracey D. Brown said.
Adult type 1 diabetes is real!
One of the few really eye-popping developments this year was the announcement of a consensus statement between American and European diabetes experts, recognizing for the first time ever that there is, in fact, such thing as adult type 1 diabetes (T1D).
Yes, nearly a quarter century after T1D was officially classified and renamed from "juvenile diabetes," medical experts have now finally issued official guidance on standards of care for adults with T1D.
The "Management of Type 1 Diabetes in Adults—2021 Draft ADA/EASD Consensus Report" is a multiyear effort between the ADA and European Association for the Study of Diabetes (EASD). It includes a new diagnostic algorithm for T1D that begins with measuring islet autoantibodies.
“We know we have guidance for the management of people with type 1 diabetes, but this gets mixed into broader guidelines and many of those broader guidelines are mostly derived from data in people with type 2 diabetes,” said Dr. Anne Peters, a well-known endocrinologist at the University of Southern California (USC) and director of the USC Clinical Diabetes Programs. “The EASD and the ADA recognized that there was a need to develop a comparable consensus report that specifically addresses the needs of people with type 1 diabetes.”
The report lays out that to achieve individualized care, patients should undergo an initial needs assessment. It also addresses behavior considerations such as alcohol and tobacco use, sleep, sick day management, driving, employment, physical activity, and nutrition.
"There is no one eating pattern recommended," said Amy Hess-Fischl, a registered dietician and nutritionist and certified diabetes care and education specialist (CDCES) at the University of Chicago. "It is all based on the individual sitting in front of us."
The report notes that there are four critical times for ongoing diabetes management support and education: at diagnosis, annually or when the patient is not meeting treatment targets, when complicating factors develop, and when transitions in life and care occur.
Dr. Jeremy Pettus, endocrinologist at the University of California, San Diego, worked in the consensus group that evaluated an array of medications that might be useful for T1D — some of them more commonly used for type 2 diabetes currently.
"There are other things wrong in type 1 diabetes physiology that we could potentially address with medications to help the vast majority of T1Ds get their blood sugars down to where they need them to be, help lose weight, improve cardiovascular outcomes," he said. "Type 1s, even with good glycemic control, are still at high risk for cardiovascular disease."
A hope is that these newer guidelines can help better diagnose T1D in varying age ranges, to help quell common misdiagnosis. But also, to further emphasize that individualized care is necessary when treating someone with the condition.
Insulin and related ‘cure’ research
Another big theme for this Scientific Sessions — and 2021 overall — was the 100th anniversary of insulin's discovery.
While so much progress has happened in diabetes and with insulin specifically since that game-changing discovery in 1921 by Drs. Frederick G. Banting and Charles Best in Toronto, the conference also highlighted how there is much left to be done for PWDs.
Affordability is at crisis levels in the U.S. and too many can't get the life-sustaining insulin they need. Yet ironically, many people with type 2 diabetes continue to live in fear of being prescribed this medication.
Sessions delved into the policy sides of insulin accessibility as well as research on new types of insulin and other islet and beta cell transplants, which fall under the "cure" umbrella.
Dr. Ruth S. Weinstock at State University of New York (SUNY) Upstate Medical University, who currently serves as the ADA's Science and Medicine division president, highlighted in her Sunday morning address that cutting-edge research is driving new therapies and technologies as well as hope for a diabetes cure. But there's a lot to be concerned about, too.
"As wonderful as the discovery of insulin was, there was a need for purer and more physiological preparations and better insulin delivery systems," she said. "We have better insulins now, but their administration is still burdensome and associated with challenges. And importantly, hypoglycemia and hypoglycemia unawareness remain problems, increasing in prevalence with longer diabetes duration."
She pointed to the price of insulin in the U.S. being higher than anywhere else in the world, and encouraged ADA attendees to work toward a goal of more affordable insulin by January 2022 — the century-mark since a 14-year-old received the first-ever dose of insulin.
Meanwhile, developments in pancreatic beta cells garnered attention at the SciSessions as a possible path toward a T1D cure.
Dr. Esther Latres of the JDRF presented updates on manufacturing insulin-producing cells from stem cells, protecting the beta cells (without immunosuppressive drugs) from being destroyed during the immune system attack on a person's body that leads to T1D.
Dr. Quinn Peterson of the Mayo Clinic presented his latest research on growing pancreatic islets from stem cells, showing findings that scientifically significant insulin production can be prompted using his technique.
As these researchers encouraged more advances in this type of diabetes research, it coincided with the recent news of President Joe Biden's proposal for a Moonshot Initiative. This would provide $6.5 billion in the federal budget for the National Institutes of Health (NIH) to fund cure-focused research on cancer and other conditions like diabetes. If that proposal gets approved and implemented, it could lead to even more T1D research on advanced treatments and a potential cure.
Time in range
Another hot topic at the ADA conference this year was the growing emphasis on Time in Range (TIR), which provides more information about glucose control than the traditional 3-month average known as the A1C.
Multiple diabetes experts in a variety of presentations highlighted the importance of TIR as they discussed latest research findings and management, complications that can materialize despite one’s A1C result, and even policy implications from looking at TIR rather than just A1C.
Generated mainly from the use of continuous glucose monitors (CGM), TIR was highlighted for how it helps people stay within the ideal 70-180 mg/dL range as often as possible in order to improve their diabetes management. This was mentioned in countless presentations and research posters.
In one of the sessions posing the question "Is CGM use an effective tool in primary care?" medical professionals and diabetes experts debated whether this tech can be useful for health consumers beyond diabetes care.
Short answer: It depends on the level of engagement a patient may have, but for those with diabetes who are dependent on insulin, the benefits of CGM are no longer in question. Presenters noted that CGM use allows a move away from focusing solely on A1C, with TIR data instead allowing healthcare providers to make better adjustments to insulin or diabetes meds, as well as determine how eating patterns or other aspects of a person's life might be tweaked to achieve better outcomes.
New weekly injectable med for type 2 diabetes
The eagerly anticipated full results of the phase 3 SURPASS trials were shared at ADA 2021, generating a lot of buzz.
The study followed up on results from early 2021 focused on tirzepatide, a new once-weekly injectable glucose-lowering combo drug (dual GIP and GLP-1 receptor agonist) from Eli Lilly. It’s still in development, but like the exciting initial results, this latest research shows the new drug leads to a sizable A1C reduction as well as weight loss and fewer hypoglycemic episodes for people with type 2 diabetes.
Diabetes complications and the ‘foot selfie’
The ADA conference also traditionally features many different research talks focused on diabetes complications. This year, there were multiple sessions aimed at kidney and cardiovascular risk for PWDs, including how various medications — especially for those with T2D — can reduce the risk of these possible complications.
There were sessions focused on spinal cord stimulation to treat painful neuropathy in the feet and toes, as well as how retinopathy is being treated more effectively now than even just a few years back.
One topic that caught our eye was “diabetes foot selfies.” Although some medical appointments to diagnosis, assess, or treat D-complications must happen in person, during the COVID-19 crisis there was a larger trend of people snapping photos of their feet and toes to have their clinicians look at those virtually to help guide decision-making.
“The COVID-19 pandemic required a rapid shift in best care practices,” said Brian M. Schmidt from the University of Michigan Medical School. “This had a huge impact on patients with diabetic foot ulcers and other complications because most of the time those patients were seen exclusively in face-to-face interactions.”
In California, Dr. Laura Shin discussed how her clinic had also used telemedicine and other methods to provide virtual care for patients with diabetes foot issues. They sent info packets to patients, families, and caregivers on conducting “three-minute foot exams,” and how to take selfies in helping clinicians prescribe care and identify high risk instances.
“A large part of us being able to treat these patients as best we could, especially with using different telemedicine technologies, was the ‘foot selfie.’ If they were flexible or agile enough, they could take the pictures themselves using their cell phones, or have a family member or caregiver take the pictures,” she said.
“With COVID-19, we have learned a lot about accessing patients,” Shin added. “Utilizing different tools and avenues for telemedicine was extremely helpful for us and for our patients with diabetes and diabetic foot care needs. And although it’s not a replacement for inpatient visits, I think we were still able to manage to keep a lot of these patients safe, keep them out of the hospital, and keep them moving in the world.”
Diabetes tech and tools on display
An anticipated highlight of the ADA SciSessions each year has traditionally been the sprawling exhibit hall, where scores of diabetes companies go all out with elaborate displays. Sales reps try to woo physicians with the latest and greatest new gadgets and tools, and many companies coordinate timing of announcements and new products with this large conference — particularly since it falls in the final month before the fiscal quarter ends and they're eager to wow investors.
Of course it's just not the same with the event being online. The virtual exhibit hall is more of a rudimentary marketing tool where you can click on materials and videos but without the fanfare and opportunity to ask questions face-to-face. But there were still some topics of interest here.
Afrezza inhaled insulin
New research was presented on MannKind's Afrezza inhaled insulin. This ultra rapid-acting inhalable drug has been available in the U.S. for adults with T1D since 2015, but it's still being studied for possible use in children and adolescents as well as for those with T2D.
In two smaller studies, MannKind showed data that Afrezza is safe in children and adults with T2D.
Researchers tested Afrezza in 30 children between 8-17 and found the inhaled insulin was safe and saw its peak action about 10-15 minutes after inhalation. Within 2 hours, it was out of their systems. For post-meal glucose drops, the children saw the peak decrease 30-60 minutes after inhalation. All of that shows Afrezza works the same in children as it does in adults. While there was a slight cough observed for some after inhalation, there was no severe hypoglycemia. This research shows a final phase 3 clinical study can now move forward, paving the way for eventual pediatric approval.
As for T2 adults, Afrezza improved their TIR throughout the day to a total 62 percent of time, or 4 additional hours each day with lower amounts of highs and lows.
Medtronic’s new products
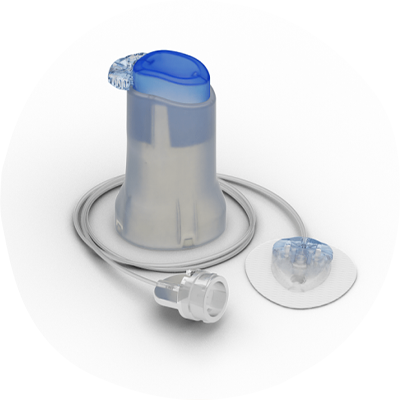
Medtronic presented important data on its future technology, including its Extended Wear Infusion Set that is already approved in Europe but is still in development for the U.S. This infusion set could last twice as long as existing infusion sets available for insulin pumps today — meaning it could be worn on the body for up to 7 days, compared with the traditional 2 or 3 days. Research presented at the ADA conference shows that Medtronic's extended wear set lasted that long for up to 75 percent of the 350+ study participants, which beat out the 67 percent for the current 2-3 day sets.
This extended wear set is already filed with the Food and Drug Administration (FDA) and is awaiting review and approval, and if OK'd it would be the first time the U.S. would see an infusion set allowed to be worn for this long.
Medtronic also presented data on Time in Range for its Bluetooth-connected 770G system, keeping up with competing diabetes device companies that presented TIR research but also setting the foundation for its upcoming 780G device (aka the Advanced Hybrid Closed Loop system) that is pending before the FDA.
With that approval, we will soon have a trio of closed loop commercial systems to choose from: Medtronic's 780G, Tandem's Control-IQ and Omnipod 5, the latter of which will be the first tubeless patch pump option with automated glucose control.
CamAPS FX closed loop system
In a clinical study from the University of Cambridge, Dr. Julia Fuchs presented data on the future CamAPS FX closed loop system in kids and teens with T1D. This technology is U.K.-based CamDiab's version of a hybrid closed loop system, combining an Android smartphone app with a Dexcom G6 CGM and an internationally available insulin pump (either the Dana Diabecare RS pump or the Dana i-pump by Korean company SOOIL).
This system adjusts insulin every 8-12 minutes based on the user's needs, with a set target glucose of 105 mg/dL. For study participants in the U.S. who didn't have access to those international pumps, the researchers used a Medtronic insulin pump and CGM. After 6 months, participants spent an average of 3.6 hours more time in range each day, or 68 percent TIR. Their A1C results also dropped by 1.1 percent, and use of the system also had other glucose-lowering benefits, they say.
- - - - - - - - - -
Originally reported by Michael Hoskins and Amy Tenderich at DiabetesMine on July 7, 2021


Comments
😊To excel in the pharmaceutical representative job search, start by obtaining a relevant degree and networking within the industry. Customize your resume and cover letter, highlighting your product knowledge and communication skills. Research companies thoroughly and consider informational interviews. Stay persistent and informed about industry trends to enhance your chances of securing a position.topmagazinepure